Serum Syndecan-1: A Novel Biomarker for Pancreatic Ductal Adenocarcinoma (#2021033)
Dr. Doron Yablecovitch & Dr. Ido Laish
Contact Us for more information:Tel Hashomer Medical Research, Infrastructure and Services Ltd.Innovation.office@sheba.health.gov.il |
| Categories | Diagnosis, Pancreatic Cancer |
| Development Stage | Proof of concept, Efficacy Studies |
| Patent Status | Pending |
Background
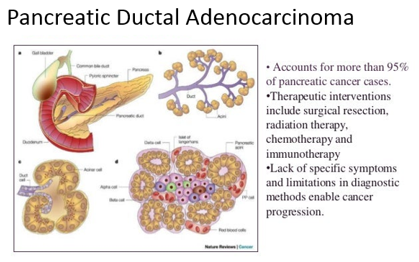
Pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer death with dismal prognosis. Despite the improvement in therapeutic strategies, the estimated five-year survival rate is only 8%, mainly because it is usually diagnosed at very late stages. This is explained by its asymptomatic nature at early stages, together with the lack of efficient screening tests for early detection. The earliest genetic event in the progression of the normal ductal epithelia to premalignant pancreatic intraepithelial neoplasia (PanIN) is the mutation of the KRAS oncogene, which functions as a driver in the tumorigenesis. Currently, the only broadly used biomarker for PDAC, CA 19-9 has multiple limitations and there is an unmet need for novel biomarkers. Liquid biopsies, including circulating tumor cells, cell-free DNA or methylated DNA, had been suggested to be used as early-stage diagnostic, as well as prognostic biomarkers, but their low-level at early stage PDAC have presented a barrier to use in diagnosis. Analyzing the metabolome is another novel approach for the detection of cancer signatures, as patients may have different metabolic profile, but there is a need for further standardizations and validation.
Syndecan-1 (SDC1) is a member of the transmembrane heparan sulfate proteoglycans (HSPGs) family, is predominantly expressed on the basolateral membrane surface of epithelial cells. SDC1 has multiple functions in tumorigenesis in general, and specifically in pancreatic cancer.
We have demonstrated that serum SDC1 is a diagnostic and prognostic biomarker in patients with pancreatic ductal adenocarcinoma (PDAC) and can be a promising novel biomarker for early blood-based diagnosis of pancreatic cancer.
The Need
PDAC frequently presents at an advanced and incurable stage of the disease. Common signs and symptoms of PDA include abdominal or back pain, jaundice, weight loss, pruritus, and nausea/vomiting. Diagnostic workup includes serum chemistries and CA19-9, primarily to monitor disease status and response to treatment. Imaging studies are performed to assess resectability and stage disease, and pancreatic protocol computed tomography (CT) scan or magnetic resonance imaging (MRI) are the preferred imaging studies for this purpose. Conventional staging is based on the American Joint Cancer Committee (AJCC) Staging System,
Pancreatic cancer is the fourth most common cause of cancer-related mortality in the United States. Biomarkers are needed to detect this cancer early during the disease development and for screening populations to identify those who are at risk.
Risk factors for developing PDAC include family history, genetic disorders, diabetes, chronic pancreatitis, and intra-ductal papillary mucinous neoplasms. An issue that hinders improvement in the prognosis of patients with PDAC is the development of a strategy to identify patients with these risk factors to facilitate detection of the disease at a stage when intervention will improve survival.
Current Diagnostic tools include:
- MRI and multidetector CT Imaging as well as pathological examination of a fine-needle aspiration from pancreatic tissue by EUS\ERCP.
- Currently , CA19-9 is the only blood-based biomarker that is being used on a routine basis and has multiple limitation with low sensitivity and low specificity
We have identified a novel serum biomarker to screen population at risk for PDAC, since patients rarely express symptoms in initial stages.
The Innovation
SDC1 is down-regulated in gastrointestinal malignancies and the loss of epithelial SDC1 has been associated with high tumor bulk, high histologic grade and shorter overall and recurrence-free survival in gastric cancer, colorectal adenocarcinoma and hepatocellular carcinoma.
However, PDAC was the only gastrointestinal malignancy in which SDC1 levels were up-regulated, and correlated with accelerated tumor growth, as determined by in situ hybridization and immunohistochemistry.
We have demonstrated the increased shedding of SDC1 to serum, caused by proteolytic cleavage by MMPs in multiple inflammatory conditions, in the setting of tissue injury and SCD1 shedding in PDAC to serum.
Serum SDC1 is elevated in patients with PDAC compared with normal individuals and patients with higher levels have a trend to reduce 1 year overall survival compare with patients with lower SDC1 levels. Its potential as a diagnostic marker can be enhanced in combination with other biomarkers, such as HPA, MMP and CA 19-9.
The Potential Products
Multiplex ELISA Kit consisting of SDC1 such as HPA, MMP and CA 19-9.
The Market
The number of new cases of pancreatic ductal adenocarcinoma is increasing with a cumulative total of 495,773 cases worldwide, making it the fourteenth most common malignancy. However, it accounts for 466,003 deaths per year and is the seventh leading cause of cancer deaths. Regional differences in the number of patients with pancreatic ductal adenocarcinoma appear to reflect differences in medical care, as well as racial differences. Compared to the prevalence of other organ cancers in Japan, pancreatic ductal adenocarcinoma ranks seventh based on the number of patients, eighth based on morbidity, and fourth based on the number of deaths, with a continuing increase in the mortality rate. The most common, pancreatic adenocarcinoma, accounts for about 90% of cases, and the term "pancreatic cancer" is sometimes used to refer only to that type. These adenocarcinomas start within the part of the pancreas that makes digestive enzymes.
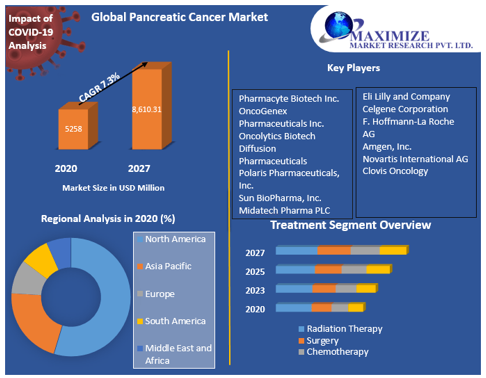
The Global Pancreatic Cancer treatment market was valued at US $5.258 B in 2020, and it is expected to reach US $8.610.31 B by 2027 with a CAGR of 7.3% during the forecast period. Pancreatic cancer is a major reason of cancer -associated mortality, with very poor whole diagnosis and prognosis that has remained virtually unchanged for many years.
The growth of the market can be attributed to the rising geriatric population around the world, increasing consumption of alcohol and smoking, and growing prevalence of obesity.
The global Pancreatic Cancer Diagnostic market size is projected to reach USD 3.887B by 2026, from USD 2.561 B in 2020, at a CAGR of 7.2% during 2021-2026.
Major players in the field include:
Roche, GE Healthcare, Siemens, BD, Philips Healthcare, Hitachi Medical, Danaher, Abbott, Canon Medical Systems, Myriad Genetics, Qiagen, Asuragen, BioMarker Strategies and ThermopFisher.
Cancer Diagnostic market, includes Tumor Biomarker Tests, Imaging, Biopsy and other products.
United States is the largest sales place, with a consumption value market share nearly 32.12% in 2017. Following United States, Europe is the second largest sales place with the consumption market share of 27.65%.
We believe that screening the population in risk via serum markers, will change the biomarker market share.
Contact Us for more information:Tel Hashomer Medical Research, Infrastructure and Services Ltd.Innovation.office@sheba.health.gov.il |