MB3 AS A DIAGNOSTIC AND TREATMENT TARGET TO INHIBIT METASTASIS IN MEDULLOBLASTOMA (#2016053)
Dr. Ruty Shai, Prof. Amos Toren, Dr. Shany Freedman
Contact Us for more information:Tel Hashomer Medical Research, Infrastructure and Services Ltd.Innovation.office@sheba.health.gov.il |
| Categories | Solid Tumor, Brain Tumor, Meduloblastoma, Metastasis, Drug, diagnosis |
| Development Stage | Pre-clinical – efficacy studies |
| Patent Status | Pending |
Background
Medulloblastoma (MB) is the most common malignant pediatric brain tumor associated with significant morbidity and mortality in addition to the neurotoxicity developing in brain caused by current chemotherapies. Metastases are the cause of death in almost every fatal case of medulloblastoma. . There are four MB tumor core subgroups: wingless (WNT), sonic hedgehog (SHH), Group 3 and Group 4.
Group 3 tumors develop high rate of metastases, which correlates with poor prognosis.
We have studied the potential pathways that are associated with Group 3 tumors, and revealed that lncRNAMB3 (MB3) may serve as a target for diagnosis and treatment for group 3 Medulloblastoma.
Long non-coding RNAs (lncRNAs) are a type of non-coding RNAs (ncRNAs), typically over 200 nt, that are abundant in the mammalian transcriptome.
LncRNAs have been shown to regulate cell functions including cell signaling, tumor progression, and metabolic regulation.
Knockdown of lncRNAs with siRNA is a useful technique to determine the function and significance of a particular lncRNA.
We have shown that MB3 inhibition by siRNA reduces invasion and migration in a cellular model. In contrast, elevated expression of MB3 induces migration, invasion and also ability of endothelial cells to form blood vessel like tubes.
The Need
Medulloblastoma is one of four brain cancers with the greatest declines (12%) in survival between 5 and 15 years after diagnosis, reflecting continued mortality related to the disease or its treatment.
Although cancer is the first cause of death in childhood, research on pediatric cancer remains limited. Moreover, clinical cancer genetic analysis typically progresses consecutively beginning with sequencing the most likely causative gene. Such tests are not informative for diseases that are not due to alteration of the genomic sequence. Therefore, in addition to known genetic syndromes, it is essential to develop new treatments targeted to long non coding RNA in the oncological clinical laboratories. Therapeutic development in these areas of pediatric brain tumors remain uniquely challenging compared to other disease areas.The medical need for therapies in in brain tumor remains especially high, and almost entirely unmet.
The current treatments including surgery, craniospinal radiation and high-dose chemotherapy have led to improvement in survival of medulloblastoma patients. However, the risk for recurrence as well as significant acute toxicities and long-term side effects of chemotherapy include infection risk, anemia, thrombocytopenia, hearing Loss, urological Complications and neurological complications (neuropathy, seizures). Moreover, radiation to the developing brain should be restricted when treating children under 6 years of age. All these underscore the urgent need for novel tumor-specific, normal brain-sparing therapies. It has also provided the incentive for research focused on providing a better understanding of medulloblastoma biology and to the identification of new therapeutic targets. Preventing Dissemination of tumor cells, augment therapy up front to kill the roots of metastasis.
The Innovation
Treatment for personalized management of medulloblastoma patients in risk for metastasis and thus predicted to have poor survival. This personalized targeted therapy is based on our discovery of the diagnostic test, which will identify the subgroup of patients that will benefit from it.
Advantages
Approximately 27% of annotated human genes encode lncRNAs. Tissue-specific expression of lncRNAs positions them as interesting potential therapeutic targets for a variety of pathologies.
siRNA has innate advantages over small molecular therapeutics and monoclonal antibody drugs because siRNA executes its function by complete base pairing with mRNA, whereas small molecule and monoclonal antibody drugs need to recognize the complicated spatial conformation with high activity, affinity and specificity. This advantage confers the siRNA modality with a shorter research and development span and a wider therapeutic area than small molecule or antibody drugs, especially for those genes that are unfeasible for development with such strategies such as long non coding RNA that do not translate to proteins.
The Potential Products
1. Diagnostic test to identify medulloblastoma patients, which are in risk of metastasis.
2. Prognostic tool to choose high-risk medulloblastoma patients and treat them more vigorously. On the other hand, spare the low risk medulloblastoma patients from unnecessary toxicities and long-term side effects.
3. Treatment to prevent metastasis.
Potential Applications
- There is a broad spectrum of options for therapeutic targeting of RNA and the growing number of approved RNA therapeutics generate significant profits.
- MB3 inhibitor for the treatment of medulloblastoma may have orphan drug designation.
- The researchers have shown that innovative MB3 inhibitor can inhibit FAK signaling at the early stages of a tumor. The MB3 inhibitor can be also an excellent target to inhibit FAK.
- MB3 inhibitor offers therapeutic potential for many other cancers (except Medulloblastoma) which FAK is substantially over-expressed, including breast, thyroid, prostate, colon, cervical, ovarian, liver and pancreatic cancer. There have been numerous efforts to inhibit FAK signaling in cancer therapy including by Novartis (preclinical TAE226) Pfizer (preclinical PF-573,228) and GlaxoSmithKline (phase I GSK-2256098).
IP Status
Application No. PCT/IL2020/050229 PCT, WO 2020/174478.
"DIAGNOSIS AND TREATMENT OF MEDULLOBLASTOMAS"
The Market
MB3 inhibitor for the treatment of medulloblastoma may have orphan drug designation.The global brain tumor drugs market was valued at about $2.4 billion in 2018 and is expected to grow to $3.41 billion at a CAGR of 9.2% through 2022.
The global pediatric brain tumor market is growing at the CAGR of ~4.1% during the forecast period and expected to reach US$ 1,659.4 million by 2023.
Cancer drugs market all set to reach USD 161.30 Billion by 2021, developing at a CAGR of almost 7.4% from 2016 to 2021.
MB3 inhibitor offers therapeutic potential for many other cancers (except Medulloblastoma) which FAK* is substantially over-expressed, including breast, thyroid, prostate, colon, cervical, ovarian, liver and pancreatic cancer.
The researchers have shown that innovative MB3 inhibitor can inhibit FAK signaling at the early stages of a tumor. The MB3 inhibitor can be also an excellent target to inhibit FAK.
On August 2018, the FDA approved the first-ever “small interfering RNA” (siRNA) product – Alnylam.
The global antisense & RNAi therapeutics market size was estimated at USD 1.09 billion in 2018 and is expected to witness a CAGR of 7.5% during the forecast period.
Investments focusing on RNA therapeutics is on the rise. Three representative mRNA therapeutic companies (Moderna Therapeutics, BioNtech, and CureVac) attracted US$2.8 billion of private investment since 2015. Since 2017, RNA- related small molecule companies have raised significant investment, including those targeting RNA directly ($262 million to Arrakis, Expansion, Skyhawk and Ribometrix) and those targeting epitranscriptomics- related proteins ($194 million to Accent, Storm, Gotham and Twentyeight- Seven Therapeutics).
There are around 400 RNA- targeting drug development programs. Of these drug candidates, 63% are in the pre- IND stage, 32% are in early- stage clinical trials (phase I or II), 3% are in phase III and 5 drugs are awaiting regulatory decisions. The largest focus for all three modalities is oncology, encompassing 22% of oligonucleotide candidates and 45% of mRNA candidates. The largest focus for all three modalities is oncology.
Bio-distribution is shaping the pipeline priorities. oligonucleotide biodistribution lipid nanoparticle approaches enable robust delivery of oligonucleotides into cells. In addition, intrathecal delivery (injection into the spinal canal or into the subarachnoid space) of oligonucleotides results in broad distribution in the central nervous system. increased 94.2% from 2015 to 2020.
RNA therapeutics have demonstrated most success in the treatment of rare diseases, especially neurological and hepatic diseases. Of the 21 late- stage RNA therapeutics, 18 have orphan status. The most commercially successful drug to date has been nusinersen, which has $4.7 billion in sales up to the end of 2019. The two currently approved siRNA drugs — patisiran (Onpattro; Alnylam) and givosiran (Givlaari; Alnylam) — target liver mRNAs. Patisiran achieved sales of more than $150 million in its first full year on the market in 2019, which are forecast to approximately double in 2020.
Trabedersen is a phosphorothioate antisense oligonucleotide (ASO) designed to specifically target human TGF-β2 mRNA which is overexpressed in many tumors. It is currently being developed for brain cancer by Mate on (Oncolitic) in phase III trial.
Novartis recently acquired the Medicines Company, the developer of cardiovascular RNA therapeutics, for $9.7 billion. This company developed the PCSK9- targeted siRNA inclisiran and TQJ230, an antisense oligonucleotide targeting LPA mRNA.
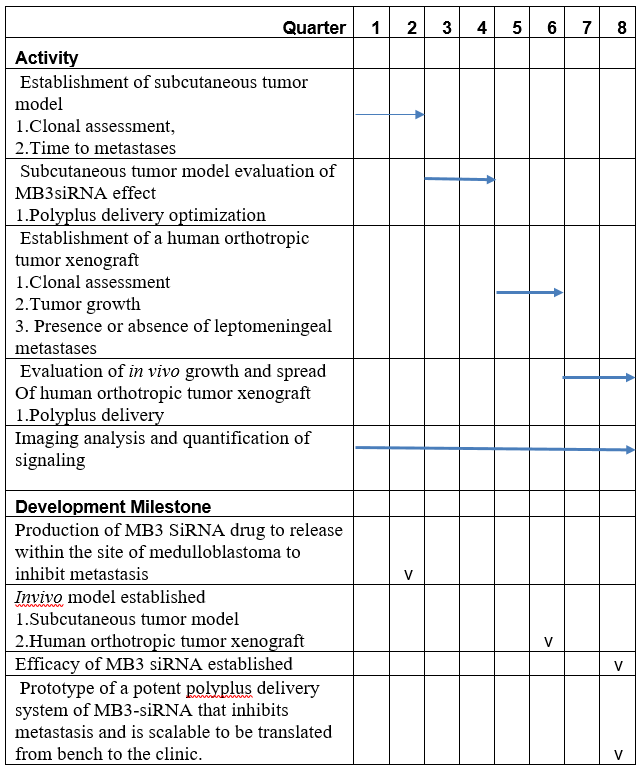
Developmemt Program
Contact Us for more information:Tel Hashomer Medical Research, Infrastructure and Services Ltd.Innovation.office@sheba.health.gov.il |