OFF-THE-SHELF T-CELLS TARGETING COMMON VIRUSES (#2020007)
Nira Blum, Elad Jacoby, Gal Cafri and Neta Zuckerman
Contact Us for more information:Tel Hashomer Medical Research, Infrastructure and Services Ltd.Innovation.office@sheba.health.gov.il |
| Categories | Cell therapy, viral infection |
| Development Stage | Conceptualization, Pre-clinical, initial efficacy |
| Patent Status | Pending |
Background
The immune system generates a cellular T-cell response to clear viral infections. These cytotoxic T-cells (CTLs), can be exploited to eliminate viruses in patients. The repertoire of T-cells recognizing viral epitopes is huge and can serve as a backbone for cell products developed specifically to eliminate common viruses. The current emerging novel SARS-CoV-2 epidemic leading to COVID-19 represents a global threat, to which there are no available preventive or therapeutic agents. There is evidence to support the role of CTLs against SARS-CoV-2 that will lead to clearance of infection and a memory response.
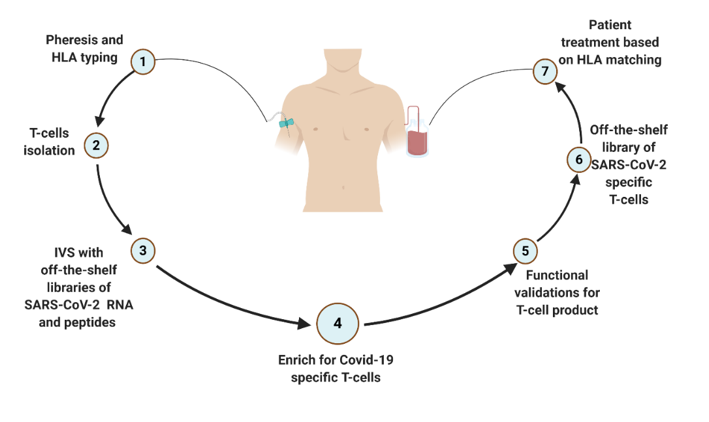
Our goal is to establishing an off-the shelf bank of anti-virus specific T cells as a therapeutic tool for common viral infections and SARS-CoV-2 . This therapeutic approach is based on priming donor mononuclear cells (MNCs) with viral peptides or mRNA to isolate and expand activated T-cells for clinical applications.
We are in the process of cloning viral specific T-cell receptors for future use in adoptive T-cell therapy. The immunological screen will also enable the discovery of new T-cell epitopes for the development of immunological biomarkers for the detection and clinical management of viral infections.
The Need
The excessive inflammatory response seen in the most serious cases of Covid-19, along with shortages of ventilators, has caused a healthcare crisis in many countries around the world.
Both common viral infections such as BKV, CMV, EBV and AAV and the current emerging novel SARS-CoV-2 epidemic leading to COVID-19 represents a global threat. There is an immense need for new and innovative therapies capable of fighting viral infections. One other huge need is to protect patients undergoing bone-marrow transplantation from common infections following treatment.
Could cell therapy provide an answer to this dilemma?
Cell therapy has shown promise for treating ARDS, which (even before Covid-19) affected 500,000 people a year across Europe, the US, and Japan.
The Innovation
Our technology involve a unique system and method to produce T cell therapy for any viral infection:
First application – cell therapy for COVID 19.
The system include:
- A bioinformatics viral antigen library
- Viral peptide library for high throughput screening of reactive T cells
T cell Libraries reactant - the of the shelf product for treatment
Capabilities of large scale manufacturing of clinical grade T-cell bank.
Advantages
- Off-the-shelf library of virus specific T-cells covering multiple epitopes
- Discovery of novel epitopes leading to new therapeutic options
- Treating multiple patients from one manufacturing process
The Potential Products
There several potential products for the project:
- Off-the-shelf clinical library of virus specific T-cells.
- Virus specific T-cell receptors for adoptive T-cell therapy applications.
- Immunological biomarkers such as viral specific tetramers for the detection of virus specific T-cells.
- Candidate epitopes for antiviral vaccines derived from the immunological analysis.
Potential Applications
- Adoptive T-cell therapy for viral diseases using virus specific cells or |T-cell receptors.
- Immunological biomarkers.
- Anti-viral vaccines
IP Status
Pending - Cell Therapy Against Viruses
The Market
- More than 5 million coronavirus cases confirmed worldwide, at least 350,000 deaths and nearly 1.6 million recoveries from COVID-19, according to Johns Hopkins University. About 1.6 million people have recovered.
- Nearly 45 million laid-off workers in the western world have applied for unemployment benefits , with economic major impact.
ICMA is carefully monitoring developments in global capital markets in response to the COVID-19 pandemic, and they are centralising a large amount of information from key resources under the following headings: Monetary policy, Regulatory responses, Market practice, Market data and commentary, Sustainable finance and Additional resources.
The information provided is not fully comprehensive given the fast-evolving environment.
Multiple therapeutic regimens are being followed across the globe in attempts to come up with a reliable treatment for Covid-19.
The contagious coronavirus is having a potential economic impact and implications on most of the sectors including the pharmaceutical industry. Governments all over the world are now responding to the threat of COVID-19 with all the essential measures such as social distancing, nationwide lockdown, travel restrictions, and large-scale quarantines that are anticipated to negatively impact the businesses and consumer spending.
Currently, there are no drugs approved by the U.S. FDA or EU for the treatment of coronavirus disease. However, the only antiviral drug approved in China is Favilavir, developed by Zhejiang Hisun Pharmaceutical. Favilavir was approved by the National Medical Products Administration of China on 18th February 2020. According to China’s Ministry of Science and Technology, favilavir is one of three drugs which demonstrated encouraging profile for blocking coronavirus in early clinical trials. The other two drugs are Gilead Sciences, remdesivir, and anti-malarial drug, chloroquine are also having promising results. 1M doses of Chloroquine from Bayer & 30M doses of hydroxycholroquine from Novartis received emergency use authorization for the treatment of Covid-19 by the U.S. FDA.
In addition, the China International Exchange and Promotive Association for Medical and Health Care (CPAM) also recommends the use of lopinavir; ritonavir in combination with nebulized alfa-interferon for the treatment of SARS-CoV-2.
Furthermore, market players are in the process of developing life-saving and life-enhancing medicines, cell therapy and vaccines for COVID-19. Several universities and key players such as Moderna , Inc.; DIOSynVax; Imperial College London, UK; Oxford University, UK; and Inovio Pharmaceuticals, Inc. are focusing on developing vaccines for coronavirus.
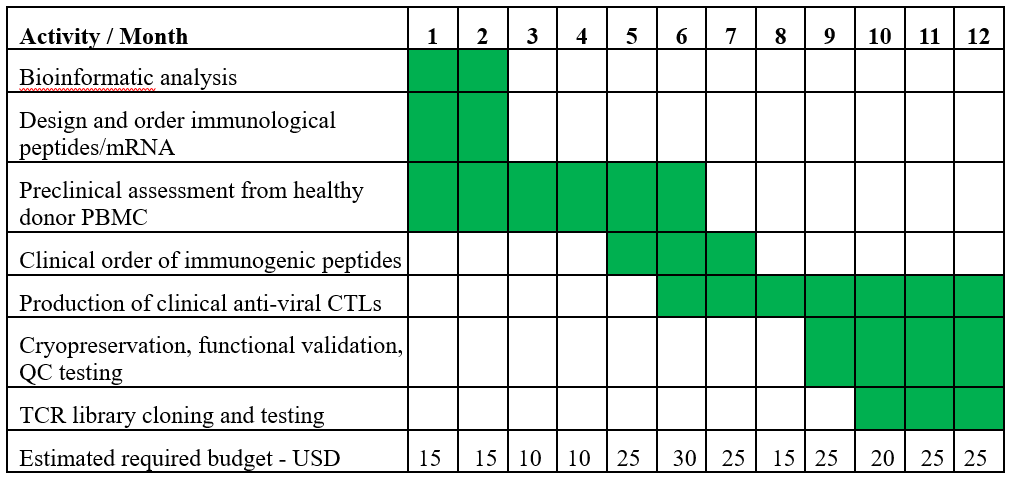
Developmemt Program
Contact Us for more information:Tel Hashomer Medical Research, Infrastructure and Services Ltd.Innovation.office@sheba.health.gov.il |