Novel Compounds for the Treatment of Liver Fibrosis/Cirrhosis (#2016045)
Prof. Ziv Ben-Ari and Dr. Michal Safran
Contact Us for more information:Tel Hashomer Medical Research, Infrastructure and Services Ltd.Innovation.office@sheba.health.gov.il |
| Categories | Therapeutic Composition , Liver Fibrosis, Fibrosis, drug |
| Development Stage | Preclinical |
| Patent Status | Pending |
Abstract
Chronic liver diseases (CLD) represent a major public health threat. Fibrosis, the most prominent feature of CLD, can progress towards cirrhosis. Cirrhosis is the major cause of liver-related morbidity and mortality causing liver failure and the development of hepatocellular carcinoma (HCC). In the normal liver, hepatic stellate cells (HSCs) constitute quiescent, vitamin A–storing cell. Upon activation by specific stimuli released by an injured liver, HSCs undergo ‘‘activation’’or trans-differentiation, yielding a myo-fibroblast-like cell. Many of the changes that cause the activation of satellite cells are caused by the secretion of proteins by neighboring cells, including liver cells (hepatocytes). Stellate cells activation is a tightly programmed response occurring in a reproducible sequence. Nevertheless, it has been demonstrated that fibrosis and even cirrhosis can be reversible if the underlying cause is successfully eliminated. Furthermore, it was demonstrated the HSCs themselves can undergo regression from differentiated myo-fibroblast like to quiescent cell.
As of today, no compound has been thoroughly validated as a therapy for fibrosis. The most effective therapy for treating hepatic fibrosis to date is still to remove the causative agent. However, this approach is suitable for the minority of patients with CLD.
In our lab, we are investigating the pathways regulating HSCs homeostasis in the healthy liver. Our results indicate for the first time that in the healthy liver, hepatocytes can inhibit directly HSCs stimulation and maintain them in their quiescent state. The same mechanism can activated the resolution of hepatic fibrosis and revert already differentiated myo-fibrobalst like cells back to their quiescent state. We have demonstrated that this mechanism is mediated by exosomes released from the hepatocytes, which carry miRNA molecules that inhibit directly the differentiation of the HSCs into active myo-fibroblasts. We have identified a specific and unique miRNA molecule that play a significant role in this process. Upon the introduction of the already identified miRNA molecule to HSCs, both in tissue culture and in in-vivo mouse model of hepatic fibrosis, we have demonstrated inhibition of the spontaneous differentiation of HSCs and regression of the hepatic fibrosis stage in-vivo. These results can facilitate the development of new potential therapeutic options that can be used to prevent the development of fibrosis and its progression toward cirrhosis.
The Need
Chronic liver diseases (CLD) represent a major public health threat affecting 1.5 billion persons worldwide, accounting for 2 million of deaths each year and imposing a high burden of morbidity. Fibrosis is the most prominent feature of CLD, and can be defined as quantitative and qualitative changes in the extracellular matrix, mainly deposition of collagen, that progress towards complete disorganization of the liver architecture; this advanced stage is known as cirrhosis. The main etiologies that underlie the development of liver fibrosis are A) Chronic viral hepatitis B or C, B) Autoimmune and biliary diseases, C) Alcoholic steatohepatitis (ASH), D) Nonalcoholic steatohepatitis (NASH).
The stage of liver fibrosis is the key determinant of prognosis in patients with CLD. Fibrosis progression toward cirrhosis, is the major cause of liver-related morbidity and mortality. Patient with cirrhosis are more prone to develop liver failure, portal hypertension and its complications and are at higher risk of developing HCC. Fibrosis and even cirrhosis is reversible in some patients if the underlying cause is successfully eliminated. However, for some patients with cirrhosis and patients which already developed HCC, liver transplantation is the treatment of choice.
Although anti-fibrotic activity has been demonstrated for many compounds in vitro and in animal models, none has been thoroughly validated in the clinic or commercialized as a therapy for fibrosis. There is an urgent need to develop anti-fibrotic therapies for CLD.
We believe that our findings will lead to the development of new compounds for the treatment of liver fibrosis/cirrhosis that will successfully limit and even reverse the progression of the disease. These compounds will allow accelerating the repair process and enhance liver regeneration in patients with CLD.
The Technology
Our main finding includes:
- We have established a unique method to isolate primary HSCs from mice livers. Using this method we have demonstrated for the first time that medium from hepatocytes can inhibit and even reverse HSCs activation.
- We have demonstrated that exosomes, extra-cellular vesicles that play a role in cell-to-cell communication, are responsible for the effect of the hepatocytes on the inhibition of HSCs stimulation.
- We have shown that in the healthy liver, exosomes secreted from the hepatocytes can bind directly to the HSCs and preserve them in a quiescent form.
- Using isolated hepatocytes secreted exosomes, we have succeeded to inhibit and even reverse the development of hepatic fibrosis in-vivo using mice model.
- We have identified a unique and specific miRNA that is secreted from the hepatocytes by the exosomes that plays a direct role in the inhibition of HSCs stimulation in-vitro and in-vivo using mouse model of hepatic fibrosis.
The Innovation
- We have developed a novel composition and demonstrated its direct activity on the inhibition and regression of hepatic fibrosis in-vivo using mouse model of hepatic fibrosis
- Novel Mechanism of action: Biological induced resolution of liver fibrosis by the direct inhibition of HSCs activation
Applications
- Hepatic fibrosis/cirrhosis unrelated to the etiology of the chronic liver disease
- Might be applicable to other organs with fibrotic tissues due to chronic diseases as lung and kidney
Advantages
- Suitable for all etiologies of chronic liver diseases causing fibrosis/cirrhosis
- A comprehensive approach- with a single compound focusing on the end-point of HSCs activation and not on a specific signal transduction pathway that is participating in the process
- Using miRNA molecules that are naturally detected in the liver, we expect no or very low toxicity
IP Status
Pending - Treatment of Liver Fibrosis by Hepatic Exosomes
The Market
Now that new medicines promise to cure millions of hepatitis C patients in coming years, drug makers are turning their attention to other chronic liver diseases than can cause liver fibrosis/cirrhosis. Cirrhosis which is estimated to affect 1% to 2% of global population results in over 1 million deaths annually worldwide. Annual direct and indirect costs for the care of cirrhosis exceed $12 billion in the U.S. alone.
The causes of CLD are numerous; most common are chronic viral hepatitis (due to hepatitis B and C viruses), alcohol abuse and non-alcoholic fatty liver disease (NAFLD), whose prevalence keeps increasing. Over the past 2 decades, NAFLD has grown from a relatively unknown disease to the most common cause of chronic liver disease in the world. In fact, 25% of the world’s population is currently thought to have NAFLD. Nonalcoholic steatohepatitis (NASH) is the subtype of NAFLD that can progress to cirrhosis, hepatocellular carcinoma (HCC), and death. The fibrosis stage is the most important independent factor associated with the disease prognosis and outcome. 10%-15% of patients with NAFLD from the United States and Europe have an advanced fibrosis stage. Because of the close association of NAFLD with type 2 diabetes (T2DM) and obesity, a 6% increase in NAFLD and a >100% increase in advanced liver diseases is predicted over the next 15 years causing a tremendous clinical and economic burden. Moreover, NAFLD and NASH are not only found in adults—there is also a high global prevalence of these diseases of 2.6% to 17.3% among children and adolescents. Nonetheless, current treatment of this disease is limited to lifestyle modifications.
Thus, antifibrotic treatments are still acutely needed, mainly in the rapidly growing NAFLD patient’s population but also in other patients with chronic liver diseases and advanced fibrosis stage with no available treatment. Reducing liver fibrosis to delay the development of cirrhosis will have a major impact on global heath worldwide and could reduce the dramatically rising economic burden. Based on the ongoing obesity epidemic in the US and its direct correlation to NAFLD prevalence, recent research by GlobalData estimates, the current market opportunity in liver fibrosis to be worth $83BN, as 70% of obese people are diagnosed with NAFLD.
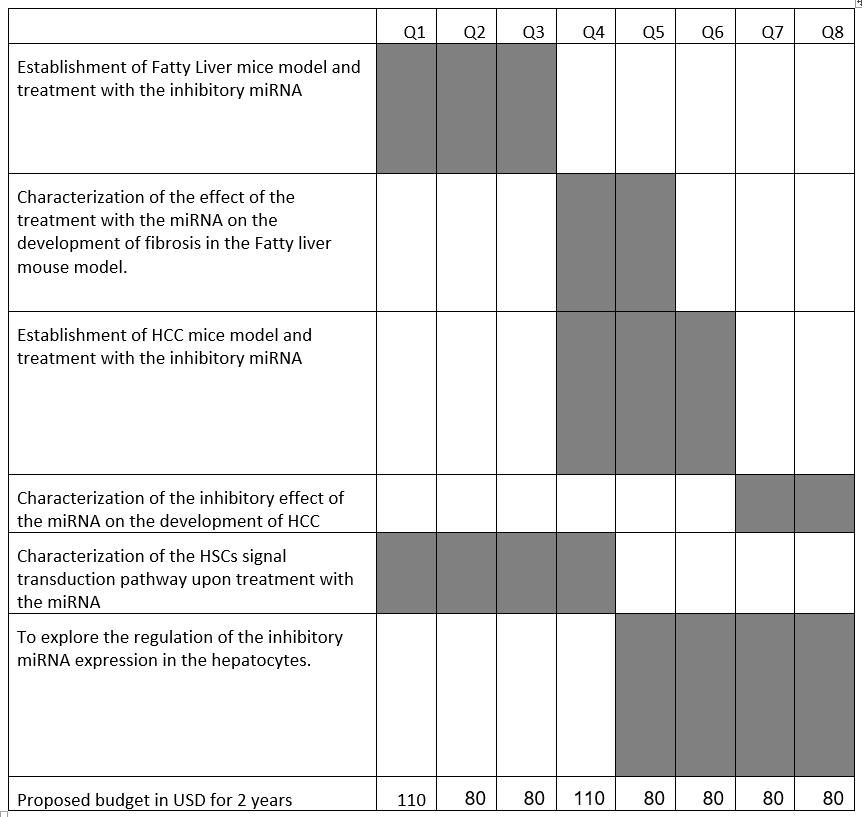
Developmemt Program
Contact Us for more information:Tel Hashomer Medical Research, Infrastructure and Services Ltd.Innovation.office@sheba.health.gov.il |