DIAGNOSTICS FOR EARLY DETECTION OF OVARIAN CANCER (#2016042)
Dr. Keren Levanon and Prof. Tamar Geiger, Sheba Medical Center and Tel Aviv University, Israel
Contact Us for more information:Tel Hashomer Medical Research, Infrastructure and Services Ltd.Innovation.office@sheba.health.gov.il |
| Categories | Diagnosis, Ovarian Cancer |
| Development Stage | Proof of concept, Efficacy Studies |
| Patent Status | pending |
Background
According to the American Cancer Society, ovarian cancer is the 8th most common cancer among women in the United States. Ovarian Cancer is the 5th most common cause of cancer deaths in women and highest rate of deaths among the gynecologic cancers. Each year, more than 22,000 women in the U.S. are diagnosed with ovarian cancer and around 14,000 will die, the overall 5-year survival rate is only 46% in most developed countries. However, if diagnosis is made early, before the tumor has spread, the 5 year survival rate is 94%. Late-stage diagnosis of high-grade ovarian carcinoma (HGOC) leads to high mortality rates. Ovarian cancer is currently diagnosed with the help of pelvic examination, blood test, ultrasound and other tests such as MRI, PET and CT. Lack of early-stage specific warning symptoms and early detection screening tests are the major causes of high rate of death due to ovarian cancer.
There are 2 tests used most often to screen for ovarian cancer, the transvaginal ultrasound (TVUS) and the CA-125 blood marker test. TVUS is a test that uses sound waves to look at the uterus, fallopian tubes, and ovaries. It can help find a mass (tumor) in the ovary, but it can't actually tell if a mass is cancer or benign. When it is used for screening, most of the masses found are not cancer. CA-125 is a protein in the blood. In many women with ovarian cancer, levels of CA-125 are high. This test can be useful as a tumor marker to follow up the treatment of women known to have ovarian cancer, because a high level often goes down if treatment is effective. Both tests fail to reduce disease-specific mortality; hence the universal recommendation for high-risk populations (e.g., BRCA1/2 mutation carriers) remains risk-reducing bilateral salpingo-oophorectomy (RRBSO) around the age of 40.
Novel approaches are developed for ovarian cancer diagnosis such as whole genome techniques to directly profile DNA methylation aberrations in cancer, identification of tumor-specific exosomes in the blood of women with ovarian cancer, intra-fallopian tube sampling, uterine washing sampling and the sampling of cervicovaginal swabs.
HGOC arise from the fallopian tube epithelium (FTE), a ‘liquid biopsy’ may be obtained through washing of the uterine and tubal cavity, a procedure termed uterine lavage (UtL). We developed a method for deep proteomic profiling of microvesicles from UtL samples, which led to definition of a diagnostic signature for detection of HGOC and disease management.
The Innovation and Development Stage
Identification of protein biomarkers in body fluids is typically hampered by the tremendous dynamic range of their proteome. To tackle this challenge, we have developed a new approach, for analysis of the proteomes of microvesicles derived from liquid biopsies, using high resolution mass spectrometry. The microvesicles are shed from all cells in our body, including cancer cells, into the extracellular space and to all body fluids. They contain a large variety of cellular proteins that represent their cell of origin and can therefore serve as an excellent source of biomarkers. Using these techniques, we measure thousands of proteins in single analyses, including very low abundance proteins which may be potential biomarkers.
Our diagnostic screening test is performed on a body fluid sample obtained from the gynecologic tract by a minimally-invasive UtL procedure. The procedure can be easily performed in the clinic with minimal inconvenience and complications.
The first patient cohort, consisting of both ovarian cancer patients and women with other benign gynecological conditions, included 54 cases. It was used to build the 21-protein diagnostic signature which was then blindly applied to an independent validation set of 106 samples, and was capable of predicting the presence of HGOC with 55% sensitivity and 84% specificity. We currently continue enrolling patients, control women and women with high risk for HGOC in order to further improve the performance of the diagnostic signature.
The Need
Ovarian cancer is characterized by few early symptoms, presentation at an advanced stage, and poor survival. As a result, it is the most frequent cause of death from gynecological cancer.
Only 20% of ovarian cancers are found at an early stage with 94% of survival rate longer than 5 years after diagnosis. Early detection and treatment should decrease mortality from this disease.
Incidence of ovarian cancer: age-standardised rates (world) per 100 000 (all ages).
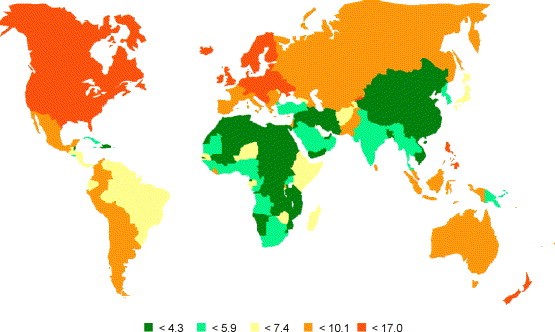
There is growing evidence that screening can impact on cancer specific mortality. Many countries have national screening programmers for breast, bowel, prostate, lung and cervical cancers with the latter associated with significant (50–90%) reduction in disease specific mortality. There is preliminary evidence that screening for ovarian cancer can improve survival, but there are currently no established international guidelines for ovarian cancer screening.
In addition, the population of women with inherited high-risk presents with a unique challenge: while the life-time risk of developing ovarian cancer is high, no screening program proved effective, and therefore women are advised to undergo a preventive surgery at age 40, even though the risk at this age is minimal and the consequences of loss of fertility and early menopause are significant. We believe that our research results may lead towards developing an early detection test for this particularly important population.
The Market
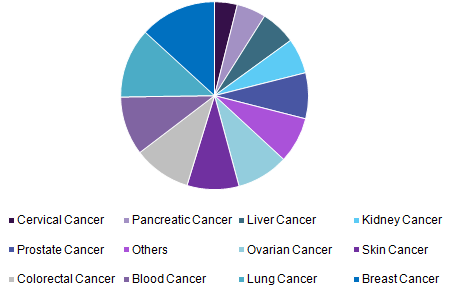
The global cancer diagnostics market size was valued at USD 124.0 billion in 2016 and is expected to grow at a CAGR of 7.2% over the forecast period to be worth up to US$168. billion by 2020.
Liquid biopsy will gradually replace surgical biopsies. New multibillion dollar market is developing for cancer screening tests. Liquid biopsy is an advanced cancer diagnostic test that uses biofluids such as blood, Cerebral Spinal Fluid, plasma, urine and more, for detecting cancer. Liquid biopsies are analyzed for specific molecules and molecular profiles of circulating nucleic acids, circulating tumor cells (CTC), exosomes and proteins as biomarker.
Global cancer diagnostics market, by application, 2016 (%)
The global liquid biopsy market revenues are projected to expand at a CAGR of 21.7% during the forecast period 2016-2026 and a reach value of US$ 2,893.7 million by the end of 2026.
Key Players are BIOCEPT, INC., Qiagen N.V., Trovagene, Inc., Janssen Global Services, LLC, MDxHealth SA, Natera, Inc., F. Hoffmann-La Roche Ltd., Silicon Biosystems, Pathway Genomics Corporation, Sysmex Corporation.
IP Status
" Diagnostic Test for Early Detection of Ovarian Cancer" Pending
Contact Us for more information:Tel Hashomer Medical Research, Infrastructure and Services Ltd.Innovation.office@sheba.health.gov.il |



